Phoenix Ambulatory Blood Pressure
Monitor Project
Sub-project: Regulatory Approval - Processes
Sub-project: Regulatory Approval - Processes
Product Introduction System
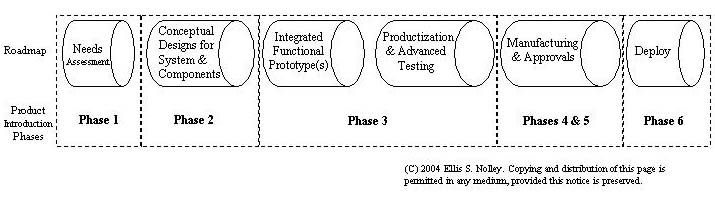
Product Roadmap (see full Roadmap)
Phase Overview
|
|
|
|
|
|
|
|||||
|
|
|
|
|
Development | |
|
|
|
|
|
Phase Description and Deliverables
About This Page
This page is maintained by Ellis
S. Nolley. It was last updated
on 28 October 2004.
The author(s) provide this information as a public service, and
agree to place any novel and useful inventions disclosed herein
into the public domain. They are not aware that this material
infringes on the patent, copyright, trademark or trade secret
rights of others. However, there is a possibility that such infringement
may exist without their knowledge. The user assumes all responsibility
for determining if this information infringes on the intellectual
property rights of others before applying it to products or services.
Copyright (C) 2004 Ellis S. Nolley. Copying and distribution
of this page is permitted in any medium, provided this notice
is preserved.